2024 ended on two significant high notes for Ryan Stowers, an assistant professor in the departments of Bioengineering and Mechanical Engineering at UC Santa Barbara. His work to explore how cells interact and are influenced by the mechanical properties of their environments, an area of study known as mechanobiology, received a boost of nearly $600,000 in combined funding from the American Cancer Society (ACS) and the National Institutes of Health (NIH).
“I’m thrilled to receive these grants from the ACS and NIH to fund our work in understanding mechanobiology of cancer and cardiovascular disease,” said Stowers, whose previous honors include a Faculty Fellowship from the Hellman Foundation, and Young Investigator awards from the Breast Cancer Alliance and the Cells, Tissue, Organs Journal. “These diseases are by far the two leading causes of death in the U.S.”
The ACS selected Stowers as a member of its inaugural class of the Catalyst Award, an honor intended to “catalyze” early-career researchers with high scoring, but not yet funded research projects either recently submitted to the ACS or the National Cancer Institute (NCI). The awards are for one year and $150,000 in direct costs, and they are intended to sustain the projects and support investigators as they continue to apply for research grants.
“We recognize that there has been a decrease in available funding for cancer research, resulting in a surplus of innovative and potentially impactful research projects that have gone unfunded, so we’re excited to be able to support these important investigations,” said Dr. William Dahut, chief scientific officer at the American Cancer Society, in an ACS press release.
Stowers’ ACS-supported project will investigate cancer cells, which are known to have chemical modifications to DNA that do not alter the DNA sequence, as a mutation does, but instead change the way the DNA is organized and accessed by cellular machinery, known as epigenetic changes. Tumor mechanics can exacerbate many aspects of cancer. However, it is not known if the mechanical properties of a tumor’s environment can also induce epigenetic changes, and if so, through what biological pathways. Through the ACS Catalyst funding, Stowers and his research group will test their hypothesis by using mechanically tunable 3D hydrogels as artificial tumor microenvironments to validate their fundings in mouse tumor models. Hydrogels are soft, water-swollen materials that can absorb large amounts of water or biological fluids without dissolving.
“Using our engineered artificial tumor microenvironments, we can test the extent to which the mechanical properties of the tumor environment specifically can drive cancer progression and metastasis through epigenetic changes,” said Stowers. “We’ll also learn how well our engineered tumor models recapitulate the complexity of tumors in the body by comparison to tumors grown in mice.”
Stowers and his co-principal investigator, Beth Pruitt, a mechanical engineering professor and chair of the Bioengineering Department, also recently received an NIH R21 Exploratory/ Developmental Research Grant from the National Heart, Lung, and Blood Institute (NHLBI). The pair will receive nearly $430,000 over two years to gain insight into cardiomyopathy, a disease that affects the heart muscle by altering the structure and stiffness of cardiac tissue. They plan to focus on cardiomyocytes, the individual muscle cells that make up the heart that contract to pump blood throughout the body, seeking to learn how the time-dependent mechanical properties of cardiac tissue, known as viscoelasticity, change in cardiomyopathy and what impact these changes have on cardiomyocytes. The R21 funding will allow the researchers to directly measure cardiac tissue viscoelasticity and then test cardiomyocyte responses using a viscoelastic biomaterial platform.
“In a paper we just published through this collaboration, we found that cardiac tissue is quite viscoelastic, and that the viscoelasticity is altered in patients that suffered heart failure,” added Stowers, who earned his PhD in biomedical engineering from The University of Texas, Austin and joined the UCSB faculty in July 2019. “We’re working to optimize a human cardiomyocyte culture platform where viscoelasticity can be varied, independent of other important mechanical and biochemical factors. Then, we can see whether viscoelastic platforms enable a more representative model of human cardiomyopathy.”
Researchers expect their results to have a positive translational impact by providing a more comprehensive interpretation of diagnostic imaging modalities, such as ultrasound elastography, by assessing viscoelasticity as a marker of disease progression. Ultimately, they hope that when combined with novel modeling techniques, their work could result in biological insights or enable identification of possible therapeutic targets.

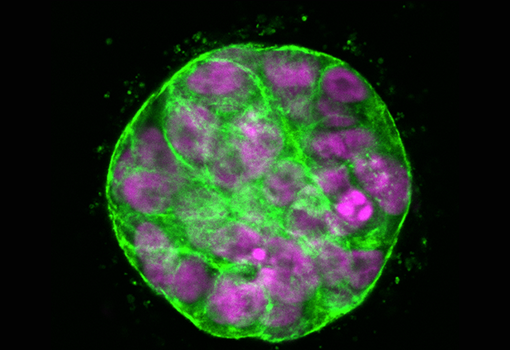
A spheroid of mammary epithelial cells encapsulated in a 3D hydrogel simulating the tumor microenvironment.
