Scientists have long sought to develop an underwater adhesive that could bind surfaces together strongly despite the barrier created by water and its impurities. Some researchers have come close, but for the most part, have fallen short of delivering a substance that performed well enough to be practical or did not require complex functionalization and processing.
Underwater adhesives are hardly a new concept. Nature provides many examples of wet adhesion, as demonstrated by barnacles and mussels, marine animals that have perfected the art of sticking to a variety of surfaces despite experiencing extremes of temperature and the constant pounding of salt water.
Taking their cue from mussels, a group of UC Santa Barbara scientists and engineers have made important progress in duplicating the mollusks’ method of generating strong adhesion. Led by research faculty member Kollbe Ahn, of UCSB’s Marine Science Institute, the researchers have successfully synthesized a material that combines the key functional molecular groups of several residues found in mussels’ biological adhesion proteins. In particular, the amino acid L-Dopa, which contains hydrogen-bonding chemical groups called catechols, shows promise.
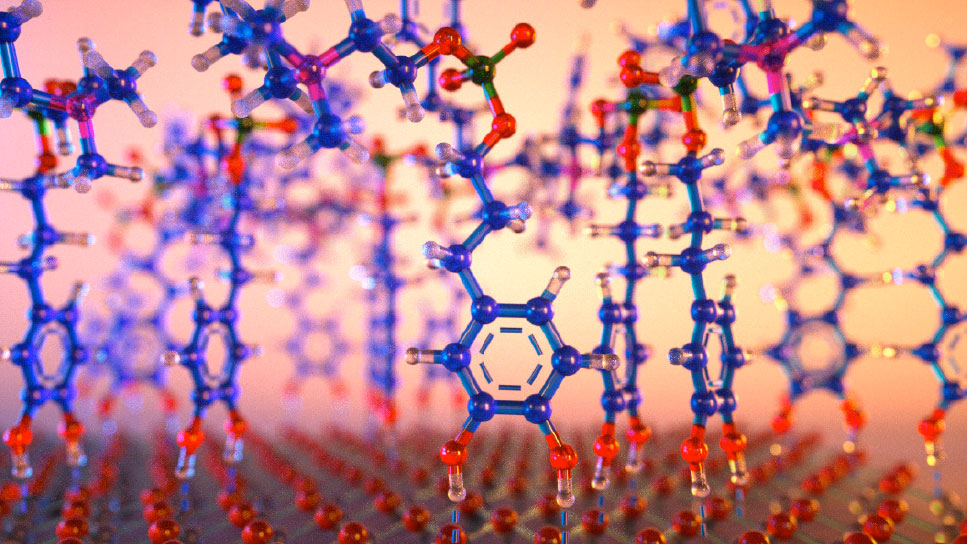 Concept illustration of a molecular-level view of the chemical reaction at the interface where a mussel's foot meets wet rock.
Concept illustration of a molecular-level view of the chemical reaction at the interface where a mussel's foot meets wet rock.Catechols are found in large quantities at the interface between the plaques at the ends of the byssus threads mussels secrete, and the often wet and submerged surfaces to which they adhere. By mimicking the characteristics of mussel foot proteins that are especially rich in this amino acid, Ahn and colleagues designed a molecule that can prime and fuse two surfaces underwater. The material, according to Ahn, is not only simple to use, but also up to ten times as effective as similar adhesives that have been demonstrated previously.
A wet adhesive could have multiple and diverse applications. In addition to the obvious — building or repairing objects that are submerged or continuously exposed to wet conditions — a wet adhesive or primer could also be added to materials so that they would self-heal in wet conditions. The material would have many uses in biomedical and dental contexts.
Using the same proteins, the scientists have also created a nontoxic primer of sub-nanometer thinness that self-assembles into a defect-free monolayer at room temperature — a potentially important development in the manufacture of polymer electronics.
Ahn credits much of this progress to the decades of research conducted by UCSB professor J. Herbert Waite, whose work investigating the adhesion strategies of mussels in the rocky intertidal zone provided the foundational knowledge of the biological adhesion mechanism at the molecular level. Further collaboration with researchers in other UCSB departments, including chemistry professor Bruce Lipshutz and colleagues in the lab of chemical engineering professor Jacob Israelachvili, was also important in making this development possible.