Monday, April 12, 2021
In the earliest stage of life, animals undergo some of their most spectacular physical transformations. Once merely blobs of dividing cells, they begin to rearrange themselves into their more characteristic forms, be they fish, birds or humans. Understanding how cells act together to build tissues has been a fundamental problem in physics and biology.
Now, UC Santa Barbara professor Otger Campàs, who also holds the Mellichamp Chair in Systems Biology and Bioengineering, and Sangwoo Kim, a postdoctoral fellow in professor Campàs lab, have approached this question, with surprising findings.
“When you have many cells physically interacting with each other, how does the system behave collectively? What is the physical state of the ensemble?” said Campàs.
Indeed, he explained, embryonic cellular tissue is a “weird material,” with each cell consuming chemical energy and using it to apply forces to its neighbors and coordinate their actions. In-vitro studies with cells in synthetic dishes provide only part of the picture, he added; by studying cells in their native environment, the living embryo, they could find out how cells control their collective state and the phase transitions that emerge from their symphony of pushes and pulls.
In a paper published in Nature Physics, Campàs, Kim and colleagues report the development of a computational framework that captures the various interactions between cells and connects them to embryonic tissue dynamics. Unlike previous simulations, this framework takes into account several key features relevant to cell interactions, such as spaces between cells, cell shapes and tension fluctuations where the cells meet.
“To fully understand the physical behavior of embryonic tissues, all key aspects of embryonic tissues at cellular scales should be taken into account in the model as emergent tissue properties derives from interactions at the cellular scale,” said Kim, the lead author of the study. “There are numerous models to study embryonic tissues, but there is no general framework that includes those key features, hindering the holistic understanding of the physical behaviors of embryonic tissues.”
Jiggling Cells
Embryonic tissue, according to the researchers, behaves physically somewhat like an aqueous foam, a system composed of individual pockets of air clumped together in a liquid. Think soap suds or beer froth.
“In the case of foam, its structure and dynamics are governed by surface tension,” Kim said. Analogous forces are found where cells come into contact with each other in embryonic tissue, on both the inner faces of the cell membranes and between cells.
“Effective forces acting on cell-to-cell junctions are governed by cortical tension and cell-to-cell adhesion,” Kim said, “so the net force at the cell-to-cell contacts can be modeled as an effective surface tension.”
However, unlike the more static forces between cells in typical foams, the forces between cells in embryonic tissue are dynamic.
“Cells in tissues do not generate static forces, but rather display dynamic pushing and pulling over time,” Campàs explained. “And we find that it is actually these tension fluctuations that effectively ‘melt’ the tissue into a fluid state.” It is this fluidity of the tissue that allows cells to reorganize and shape the tissues, he explained.
The researchers put their model to the test by measuring how forces change over time in embryonic zebrafish, a popular model organism for those studying vertebrate development. Relying on a technique developed in the Campàs Lab using tiny magnetic droplets inserted between cells in embryonic zebrafish, they were able to confirm, by the way the droplet deformed, the dynamic forces behind the fluid state of the tissue.
Their finding that tension fluctuations are responsible for the fluidity of tissue during development stands in contrast to the generally accepted notion that changes in adhesion between cells is the critical factor that controlled the fluidity of the tissue — if the adhesion between cells reached a certain high threshold, the tissue would become fluid.
“But since cell forces and tensions fluctuate in embryos, it could be that these played an important role in tissue fluidization,” Campàs said. “So when we ran the simulations and did the experiments, we realized that actually the jiggling was way more important for the fluidization than the adhesion.” The fluid state of the tissue is the result of the dynamics of forces, rather than changes in static cell tension or adhesion.
The findings of this study could have implications in the field of physics, particularly in the realm of active matter — systems of many individual units that each consume energy and apply mechanical forces that collectively exhibit emergent collective behaviors. The study could also inform studies in biology, in investigations of how changes in individual cell parameters could control the global state of the tissue such as with embryonic development or with tumors.
Research in the study was conducted also by Marie Pochitaloff and Georgina A. Stooke-Vaughan at UC Santa Barbara.
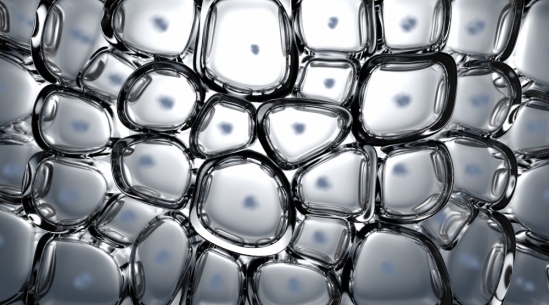
Cells resemble foam in the earliest stage of development.
